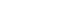 The SSRL Structural Molecular Biology program operates as a integrated resource and has three primary areas (or cores) of technological research and development and scientific focus: macromolecular crystallography (MC), x-ray absorption spectroscopy (XAS), and small angle x-ray scattering/diffraction (SAXS). Central to the core technological developments in all three of these areas is the development and utilization of improved detectors and instrumentation, especially to be able to take maximum advantage of the increasingly high brightness of SSRL’s storage ring (SPEAR3). There is also research and development in new methods - in techniques and instrumentation development and deployment. Included is the use of enhanced computing and data management tools to provide more "user-friendly, real-time and on-line" data reduction and analysis. The innovations in beam line, instrumentation and methodology developments are guided by a very close coupling to the collaborative projects aimed at solving forefront problems in SMB in all three core areas. There is significant synergy between the three core areas and with the collaborative projects to address increasingly complex and challenging problems. The SMB program seeks to sustain and enhance the general user program through excellent support, training and dissemination. More information on each of the specific core areas can be reached at the links found below.
The SSRL Structural Molecular Biology program operates as a integrated resource and has three primary areas (or cores) of technological research and development and scientific focus: macromolecular crystallography (MC), x-ray absorption spectroscopy (XAS), and small angle x-ray scattering/diffraction (SAXS). Central to the core technological developments in all three of these areas is the development and utilization of improved detectors and instrumentation, especially to be able to take maximum advantage of the increasingly high brightness of SSRL’s storage ring (SPEAR3). There is also research and development in new methods - in techniques and instrumentation development and deployment. Included is the use of enhanced computing and data management tools to provide more "user-friendly, real-time and on-line" data reduction and analysis. The innovations in beam line, instrumentation and methodology developments are guided by a very close coupling to the collaborative projects aimed at solving forefront problems in SMB in all three core areas. There is significant synergy between the three core areas and with the collaborative projects to address increasingly complex and challenging problems. The SMB program seeks to sustain and enhance the general user program through excellent support, training and dissemination. More information on each of the specific core areas can be reached at the links found below.
Macromolecular Crystallography (MC)
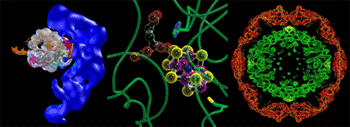
 The macromolecular crystallography (MC) program is a major experimental driver for structural biology research, serving the needs of a large number of academic and biotech groups working in this area, particularly in the Western U.S. Innovations in synchrotron-based crystallography have been catalyzed by challenges in this field, in particular the growing number of “non expert” user groups and the development and application of new approaches to handle challenging and demanding classes of problems. Solving the phase problem using multi-wavelength anomalous dispersion phasing (MAD) for increasingly large proteins, improving the resolution of large structures with high throughput screening coupled to high quality monochromatic data collection, studying the most challenging biomolecular problems (larger macromolecular complexes with large unit cells, smaller “micro” crystals, and more mechanically and radiation sensitive samples), acquisition and automated approaches to “pipelining” structure determination – all continue to strongly drive the strategy for future developments and in turn give rise to increased user demand and scientific productivity. For more information on beam lines and resources, please go to the Macromolecular Crystallography www pages
The macromolecular crystallography (MC) program is a major experimental driver for structural biology research, serving the needs of a large number of academic and biotech groups working in this area, particularly in the Western U.S. Innovations in synchrotron-based crystallography have been catalyzed by challenges in this field, in particular the growing number of “non expert” user groups and the development and application of new approaches to handle challenging and demanding classes of problems. Solving the phase problem using multi-wavelength anomalous dispersion phasing (MAD) for increasingly large proteins, improving the resolution of large structures with high throughput screening coupled to high quality monochromatic data collection, studying the most challenging biomolecular problems (larger macromolecular complexes with large unit cells, smaller “micro” crystals, and more mechanically and radiation sensitive samples), acquisition and automated approaches to “pipelining” structure determination – all continue to strongly drive the strategy for future developments and in turn give rise to increased user demand and scientific productivity. For more information on beam lines and resources, please go to the Macromolecular Crystallography www pages
X-ray Absorption Spectroscopy (XAS)
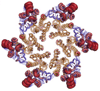
 Metal ions have key roles in biological structure and function - from being active sites of many enzymes to shuttling electrons in key metabolic pathways, having roles in signaling pathways and being key elements of cancer chemotherapies and disease-related biological malfunctions. Structural information on metal sites in biomolecules can be obtained from x-ray absorption edge and extended fine structure (EXAFS) experiments (collectively called XAS). XAS spectromicroscopy provides spatially resolved information about metal distribution and speciation in materials of biological and medical relevance, including tissues. The SSRL SMB Center has developed one of the largest dedicated and concentrated activities in the world with optimized beam lines and specialized instrumentation and analysis capabilities for enabling such applications. Specialized instrumentation also provides for investigation using XAS in combination with macromolecular crystallography. For more information on beam lines and resources, please go to the X-ray Absorption Spectroscopy www pages.
Metal ions have key roles in biological structure and function - from being active sites of many enzymes to shuttling electrons in key metabolic pathways, having roles in signaling pathways and being key elements of cancer chemotherapies and disease-related biological malfunctions. Structural information on metal sites in biomolecules can be obtained from x-ray absorption edge and extended fine structure (EXAFS) experiments (collectively called XAS). XAS spectromicroscopy provides spatially resolved information about metal distribution and speciation in materials of biological and medical relevance, including tissues. The SSRL SMB Center has developed one of the largest dedicated and concentrated activities in the world with optimized beam lines and specialized instrumentation and analysis capabilities for enabling such applications. Specialized instrumentation also provides for investigation using XAS in combination with macromolecular crystallography. For more information on beam lines and resources, please go to the X-ray Absorption Spectroscopy www pages.
Small Angle X-ray Scattering and Diffraction (SAXS)
 X-ray scattering from solutions or partially ordered arrays of biomolecules typically provides low-resolution (~7-10 Å or higher) structural information. Such studies can be done in solution, are relatively fast and require small quantities of material. SAXS studies are well-suited for time resolved measurements and hence can be used to address questions like conformational changes or folding intermediates under more biologically relevant conditions than available in, for example, crystals. Increasingly, structures of large complex systems determined by SAXS at low resolution, when combined with high-resolution structures of individual components determined by macromolecular crystallography, have been used to gain more detailed insight into very large, complex assemblies. The SMB Center provides one of the few dedicated beam lines in the U.S. devoted to solution SAXS and fiber diffraction studies and the only one that is part of an integrated Center, facilitating combined MC, XAS and SAXS studies. For more information on beam lines and resources, please go to the Small Angle X-ray Scattering and Diffraction www pages.
X-ray scattering from solutions or partially ordered arrays of biomolecules typically provides low-resolution (~7-10 Å or higher) structural information. Such studies can be done in solution, are relatively fast and require small quantities of material. SAXS studies are well-suited for time resolved measurements and hence can be used to address questions like conformational changes or folding intermediates under more biologically relevant conditions than available in, for example, crystals. Increasingly, structures of large complex systems determined by SAXS at low resolution, when combined with high-resolution structures of individual components determined by macromolecular crystallography, have been used to gain more detailed insight into very large, complex assemblies. The SMB Center provides one of the few dedicated beam lines in the U.S. devoted to solution SAXS and fiber diffraction studies and the only one that is part of an integrated Center, facilitating combined MC, XAS and SAXS studies. For more information on beam lines and resources, please go to the Small Angle X-ray Scattering and Diffraction www pages.
Acknowledgement
The SSRL SMB Resource supports the development of advanced methodologies and research, collaborative research, service, training and dissemination in structural molecular biology using synchrotron radiation at SSRL. The integrated program is supported primarily by the NIH NCRR through a Research Resource P41 grant, by the DOE Office of Biological and Environmental Research, and by the NIH National Institute of General Medical Sciences. Publications deriving from the use of this Resource must provide appropriate citations to the supporting agencies.
![]()