Utrafast Chemical Science ProgramOverarching
science goal:
The
Ultrafast Chemical Science Program at the SLAC National Accelerator
Laboratory focuses on
ultrafast chemical physics research at SLAC that is enabled by LCLS,
the
world’s first hard x-ray free-electron laser. The on-site presence of LCLS with
its facilitating connections to our research is one of two
distinguishing
advantages of this FWP within the BES program.
Our other distinguishing
advantage is our close connection to the
research powerhouse of Stanford University, through its Stanford PULSE
Institute. These help to keep us
competitive
on a national and international level. Funding
Source:
We are funded by the
Atomic,
Molecular, and Optical Sciences
Program in the Chemical
Sciences, Geosciences and Biosciences Division of Basic
Energy Sciences in the Office of Science, Department of Energy. Jeffrey
Krause is our DOE Program Manager. Major themes: • Imaging on the
nanoscale in space and the femtoscale in time.
Microscopy at its
most
essential level in
both space and time is paramount to the BES mission to control matter.
Non-periodic nano-structures and ultrafast timescales dominate the
workings of
biology and chemistry.
To understand and
control function we therefore must first observe structure and motion
on these
scales. LCLS is a revolutionary
x-ray source for investigations on
the nanoscale, and our largest subtask is devoted to developing science
using
coherent x-ray imaging techniques at this and other x-ray free electron
lasers.
This work includes
nanocrystal
imaging on the few-Angstrom scale; nonperiodic imaging of single
biomolecules;
cell imaging; and imaging of aerosols. • Light
conversion
chemistry.
Light from the sun
is the
primary source of energy on earth,
and so we are exploring its conversion to electron motion and then to
chemical
bonds. Some molecules are particularly adept at this conversion and we
would
like to understand how they work. For
example, we are especially interested in the process of photocatalysis
within
coordination complexes and similar materials.
Energy conversion is
initiated by charge separation, and we
know that the charge distribution of the electron and hole, as well as
the
presence of low energy ligand field excited states greatly influence
the
lifetime of optically generated charge transfer excited states. Still,
the detailed mechanism for the excited
state quenching remains unclear. New
methods of linear and nonlinear spectroscopy, and especially x-ray
spectroscopy
involving short-pulse FEL’s, can help provide the answer. • The eV scale in
time, space, and field strength. This is the fundamental
scale that determines structure and
dynamics of electrons in molecules, and motivates advances in
sub-femtosecond
time-resolution and Angstrom spatial resolution in theory and
experiments.
To achieve an adequate view
of the molecular
realm with at this level, we must interrogate atoms with fields
comparable to
Coulomb binding fields, and on time scales set by the electronic energy
splittings in atoms.
One method to reach this
scale is through high harmonic
generation.
We will extend our use of
this technique to higher energies and with greater control over the
target
molecule, to interrogate the detailed motion of the electrons. We are
particularly interested in coupled motion of multiple electrons, that
goes
beyond the “single active electron” approximation
that has dominated thinking
about strong field laser-atom physics.
New theoretical approaches
are also required for this, and we are
tackling these as well. Professor
Philip Bucksbaum, AMO
Physics, Program Spokesperson; Associate Professor
David Reis, Nonlinear x-ray science, Deputy Spokesperson; Assistant Professor
Kelly Gaffney, Physical Chemistry; Professor Todd
Martinez, theory;
Research Scientist
Dr. Mike Bogan, biochemistry; Senior Research Scientist
Dr. Markus Guehr,
AMO science.
Space allocations: Most of our research activities take place in renovated space in the SLAC Building 40a Central Laboratory
Subtasks: These six key personnel
are responsible for six subtasks, which
represent six different areas of
expertise:
1. UTS: Ultrafast Theory
and Simulation (Martinez) 2. ATO: Attoscience
(Bucksbaum, Guehr) 3. SPC: Ultrafast
Chemistry (Gaffney) 4. NPI: Non-periodic X-ray
Imaging (Bogan) 5. SFA: Strong Field AMO
Physics (Bucksbaum) 6. NLX: Strong Field and
Nonlinear X-ray Optical Science
(Reis) The
broad backgrounds of our PIs provide
needed synergy for effective collaborations in cross-disciplinary
projects. Support
operations (finance, HR, safety,
purchasing, travel) are directed by the Photon Science Associate
Laboratory
Director and the Chemical Sciences Director and their staff. They
provide oversight and delegate the work
to appropriate offices in the SLAC Operations Directorate or to the
staff of
the Stanford PULSE Institute.
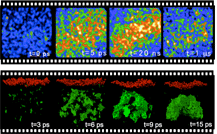
Raw snapshots of the X-ray scattering from an
optically-excited
semiconductor near the ablation threshold as a function of time.
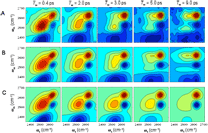
2-D vibrational correlation spectra of the OD
stretch in an
isotopically mixed aqueous solution of 6M sodium perchlorate collected
at a series of waitint times, Tw.

 |

 enlarge
this image
enlarge
this image