About Stanford EMSI
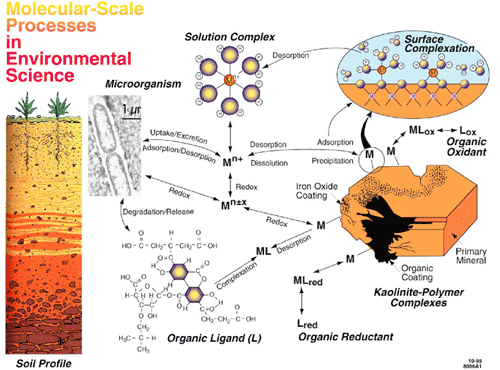
Stanford EMSI’s focus is chemical and microbiological interactions at solid-aqueous solution interfaces in Earth’s near-surface environment, where natural waters, natural organic matter, and biological organisms interact with natural solids and environmental contaminants. Although the field of surface chemistry is approaching 200 years old, rudimentary models dominate the molecular description of solid-aqueous solution interfaces and abiotic and biotic environmental interfacial reactions. Such reactions have played key roles in shaping the geosphere and biosphere of Earth throughout much of geologic time and now help mediate anthropogenic impacts on the environment. Molecular-level understanding of environmental interfaces is needed to help mitigate these impacts through better predictive models and improved remediation strategies.
Our unique, interdisciplinary approach takes advantage of recent advances in diverse molecular-level methods to address the complex interactions of aqueous inorganic species and microbial organisms with solid surfaces at a fundamental level unachievable a few years ago. We use synchrotron radiation-based spectroscopy and microspectroscopy, modern computational chemistry, and molecular genomics to study the interactions of environmentally abundant and reactive mineral surfaces, water, common heavy metal and metalloid contaminant ions, and common soil bacteria. The complexity of chemical and microbial processes taking place at environmental interfaces dictates a collaborative, interdisciplinary approach. The EMSI team of researchers brings relevant expertise in aqueous and surface geochemistry, biomineralization, computational chemistry, molecular microbiology, physical chemistry, soil chemistry, and surface chemistry and physics. Our team also has expertise in relevant in situ surface-sensitive synchrotron techniques and in scanning tunneling and atomic force microscopy.
The Stanford EMSI on Chemical and Biological Interactions at Environmental Interfaces grew out of an NSF Collaborative Research Activity in Environmental Molecular Science (CRAEMS) grant at Stanford University on a similar topic that was funded from 2000-2005 by the NSF Chemistry Division. The original investigators in the NSF-CRAEMS grant [Gordon Brown, Scott Fendorf, Anders Nilsson, and Alfred Spormann (all Stanford University) and Satish Myneni (Princeton University)] formed the nucleus of the Stanford EMSI effort. This was expanded significantly to include new expertise in chemical physics [Uwe Bergmann, Kelly Gaffney, Mike Toney (all at Stanford Synchrotron Radiation Laboratory) and Kevin Rosso (Pacific Northwest National Laboratory)]; computational chemistry [Anne Chaka (National Institute for Standards and Technology)]; and state-of-the-art synchrotron radiation methods [(Hendrik Bluhm and Miquel Salmeron (both at Lawrence Berkeley National Laboratory) and Tom Trainor (University of Alaska, Fairbanks)].
EMSI has a major educational outreach component that includes summer science teacher training workshops, summer undergraduate research opportunities, and a summer school on applications of synchrotron radiation. The educational outreach effort is lead by Prof. Bryan Brown of the Stanford School of Education and Dr. Jennifer Saltzman, Educational Outreach Coordinator for the Stanford School of Earth Sciences.
Major funding is provided by the NSF Chemistry Division, and additional funding comes from the NSF Earth Sciences Division. Some of the national lab members of the Stanford EMSI receive funding from the DOE Office of Biological & Environmental Research, Environmental Research Science Division.
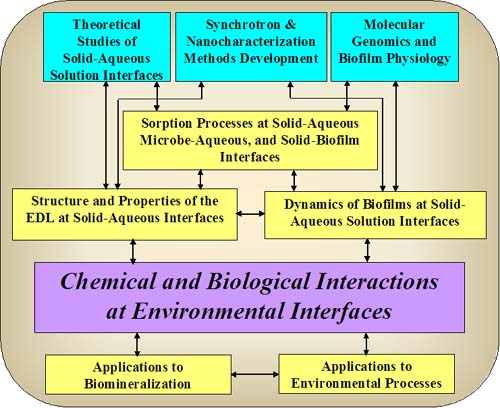
Stanford EMSI Research Outline
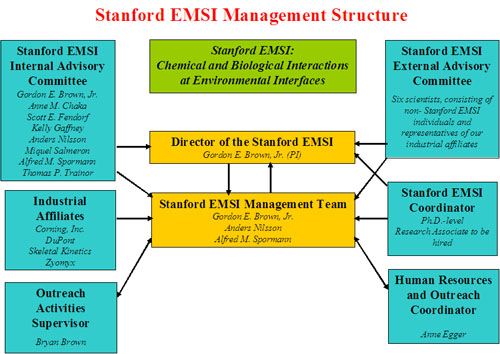
Stanford EMSI Management
Stanford EMSI Goals
-
To develop a quantitative molecular-level understanding of the chemical and biological processes occurring at environmental interfaces and how they affect pollutant speciation, toxicity, mobility, and potential bioavailability
-
To explore how such interactions studied in the laboratory relate to the complexity found in natural environments
-
To provide platforms for new approaches to address environmental challenges involving pollutants
-
To recruit a diverse group of qualified graduate, undergraduate, and postdoctoral students, particularly women, under-represented minorities, and people with disabilities, to help conduct the proposed research
-
To create a stimulating multidisciplinary research/learning environment in which students and post-Ph.D. participants can tackle complex systems and questions relevant to problems in environmental chemistry, ranging from molecular to field scales
-
To effectively disseminate our research results to the broader public and to future generations of scientists, engineers, and policy makers and to engage K-12 science teachers in current topics in environmental chemistry








